DentoClude™ F is a USFDA approved novel/proprietary finely ground bioglass indicated to use as a desensitizing agent for dentin surfaces by blocking dentin tubules to help prevent micro-leakage and to use under direct or indirect restorations following dentin etch and prior to dentin adhesive application.
Inlays and on lays are cemented in the mouth using adhesive resin luting cements. These materials are placed in the inlay/ on lay and placed onto the prepared tooth. Bonding agents can be applied to dentine before cementation of inlays and on lays.
DentoClude™ F can be used immediately after self-etchant adhesive as the dental bonding composition dissolves the smear layer, demineralizes the dentin to expose dentin matrix and primes the etched dentin surface. In addition, the bioactive glass and adhesive penetrate the demineralized dentin to form the hybrid layer. Then the dental bonding can be cured.
Data supporting the safety and efficacy of ingredients of DentoClude™ F is available in public domain. Data can be provided upon request.
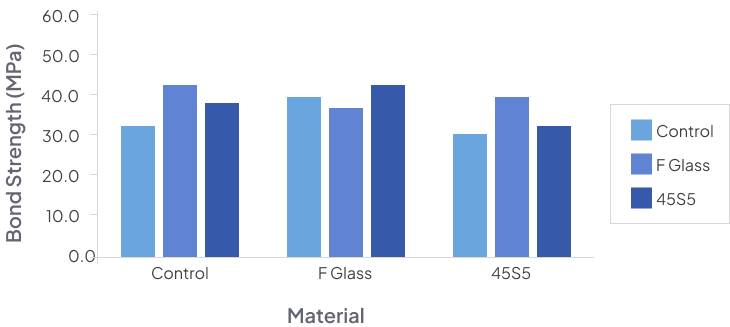
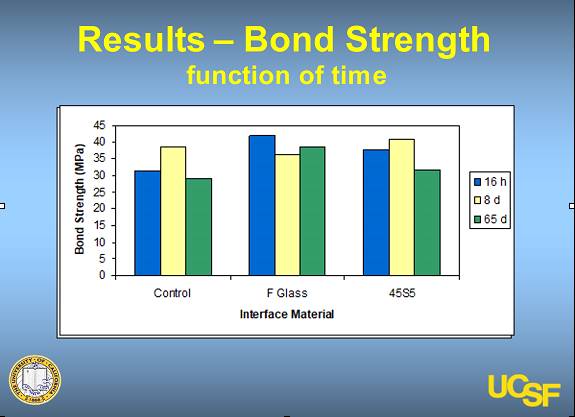
In-Vitro and In-Vivo studies were conducted to test the efficacy and viscosity of DentoClude™ F and all of them demonstrated positive results with no deleterious effects on bond strength.
Zamet JS, Darbar UR, Griffiths GS, Bulman JS, Brägger U, Bürgin W, Newman HN. Particulate bioglass as a grafting material in the treatment of periodontal intrabony defects. J Clin Periodontol. 1997 Jun;24(6):410-8. doi: 10.1111/j.1600-051x.1997.tb00205.x. PMID: 9205920.
Ette S. Tadjoedin, Gert L. De Lange, D. M. Lyaruu, Luit Kuiper, Elisabeth H. Burger. High concentrations of bioactive glass material (BioGran®) vs. autogenous bone for sinus floor elevation. Clinical Oral Implants Research
DentoClude™ F is priced as premium per the cost and value based pricing with an intent to bring an efficacious product on to the market to address one of the most prevailing dental health issue, Dentinal Hypersensitivity.
The intent to market the DentoClude™ F as a single use is to ensure reduced risk of contamination. If accidentally opened and need to reuse in a day, ensure DentoClude™ F to remain in the original well as provided, seal back and refrigerate.
At DentoClude™ F, we innovate dental solutions to enhance patient care. Our flagship product, DentoClude™ F, offers cutting-edge technology for effective tooth sensitivity relief.
© 2024, DentoClude™ F, All Rights Reserved, Powered by Kerlotech.